As the world marshals its collective resources in the fight against COVID-19, an often forgotten major Public Health accomplishment achieved through an intense global Public-Private Partnership is worth revisiting.
What if someone had suggested a year ago to imagine a world where Public Health matters more than anything else? A world where every human being and society would be actively discussing and engaging – on a daily basis – social responsibility and community health.
Partners in Progress Series
That world would have seemed improbable. And yet here we are, engaging COVID-19 as part of our daily routine.
Not only is Public Health top of mind, but the collective global effort and pace to discover a solution is unprecedented. We all watch and wonder – as both public and private enterprises work toward a common Public Health cause – how quickly a solution can be found. Was there ever a time in Public Health’s history where an infectious disease had this level of global awareness and response? And if so, what can we learn from how it was handled by the Public Health community?
One such example stands out as being legendary – where the collective force of emerging technologies, social responsibility, mobilization, health policy, public-private partnerships, and behavioral change – transformed millions of lives in Africa dramatically.
This story also marked the beginning of a revolution based on integrated, multi-disciplinary, biologically-based interventions – a prime example of Public and Private partnerships, and Public Health achievement on a grand scale”
Unseen Enemy
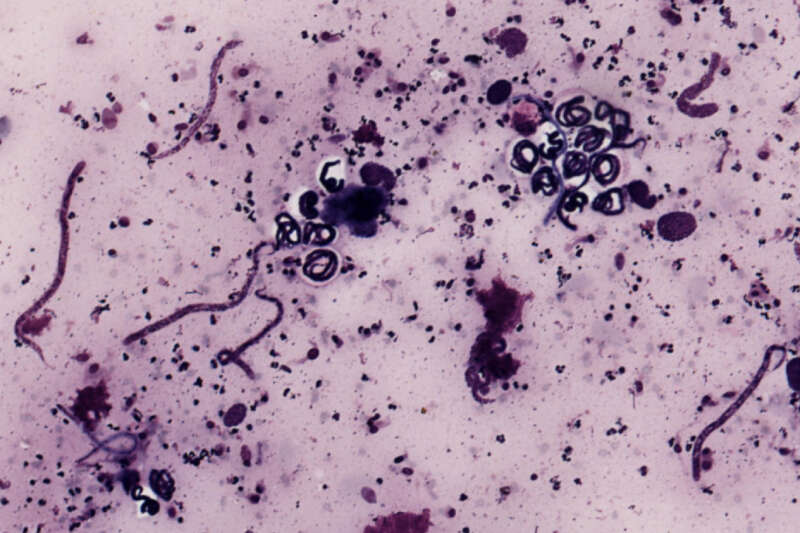
Imagine now that you’re an eight year-old in West Africa in the mid-1970s. Considering you live in an impoverished, developing country, perhaps it doesn’t seem strange to you that more than half the men in your village – 40 years old or older – are blind. It does seem strange to you – and more than a little bit scary – that they are blind from worms that crawl around the inside of their eyes.
Onchocerca volvulus is a parasitic (filarial) worm that is part of the helminth group. As much as one-third of the world’s population is said to be afflicted with helminthic parasites. O. volvulus causes onchocerciasis, or River Blindness, a devastating disease of which 99% of all cases take place in Africa. Millions of people are affected by this parasite annually, including a few pockets of infection in Central America where the parasite migrated during the slave trade.
By the 1920s, it had been known that various species of Simulium (a genus of black flies) are an intermediate host for O. volvulus parasites that constitute the disease. Black flies breed in rapidly flowing streams and rivers.
In the late 1940s and 1950s, onchocerciasis took a beating as the Public Health community attacked it with a powerful new arsenal of post-war synthetic chemistry. Using DDT, temephos and other new compounds, governments were highly successful in onchocerciasis vector control efforts by treating West African rivers for Simulium. An onchocerciasis larviciding program was launched in Kenya in 1946, and by 1955, it had successfully interrupted the onchocerciasis disease cycle.
While it seemed as though the challenge was solved, a harsh reality was looming. While this first attempt taught us that control of the disease through effective vector management was possible, it also taught us how an avalanche of undermining factors can suddenly change everything.
By the early 1970s, Rachel Carson and Silent Spring had boldly challenged decades of pest control philosophy and operations. It was no coincidence that at the same time, reports surfaced that fish were dying in the rivers that were being larvicided with organophosphates and DDT for Simulium. Moreover, the powerful synthetic insecticides that had been unleashed against black fly were not working as they had been. It was obvious to scientists that widespread insecticide resistance was setting in.
That meant back to square one. After twenty years of successful vector management efforts, West Africa was losing the battle to control their black fly populations – and subsequently onchocerciasis . And at that point in history, our dependence on larviciding was absolute. Vector control was the only onchocerciasis intervention available.
Horrifying Results
When the World Health Organization (WHO) arrived to survey endemic West African villages in in the early 70s, the scene was dramatic.
With no effective control measures in place over the span of several years, acute onchocerciasis had steadily crept back into the African river valleys. For our imaginary eight year-old, his once prosperous village had been reduced to a hobbling mass of the afflicted. The fertile agricultural land that had made his village prosperous for generations now lay choked, overgrown, and gone to seed.
WHO workers found that in the affected savannah villages, more than 60% of the population were infected with the parasite. 10% of the adult population was blind and 30% was visually impaired. An estimated 50% of the males 40 years and older had been blinded by the disease. Because of its debilitating effects on productivity, education, development, and quality of life, onchocerciasis was a disease firmly linked to poverty.
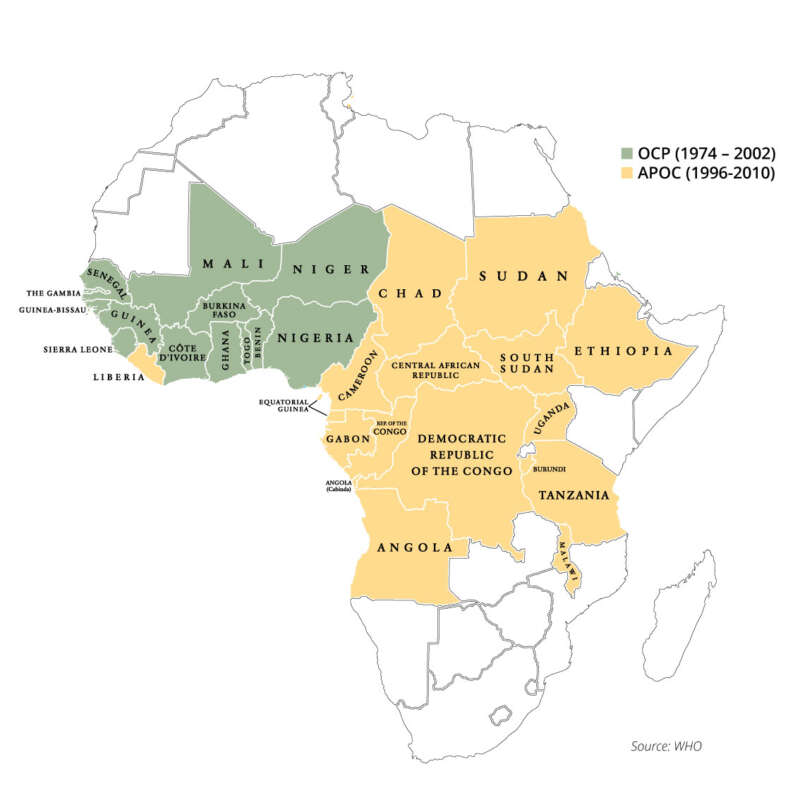
To address this tragedy, the historic “River Blindness Partnership” was created in 1974 between the World Bank, World Health Organization (WHO), Food & Agriculture Organization (FAO), and the United Nations Development Program (UNDP). Officially named the Onchocerciasis Control Programme (OCP), operations would be conducted first in seven West African countries, then later expanded to 11. In 1996, a second, more expansive program (APOC – the African Programme for Onchocerciasis Control) would expand the number of African countries to 30.
When a black fly bites a human being infected with the parasite, it ingests microfilariae through its bloodmeal. The juvenile then further develops in its new environment and grows to sexual maturity within a week. Once this is accomplished, the sexually active worm returns to a human host through another bite, where it eventually matures into an adult, completing the life cycle.
This was the setting for one of the most legendary Public-Private Partnerships in Public Health history, one that set a new precedent for the possibilities and use of sustainable, biological control.
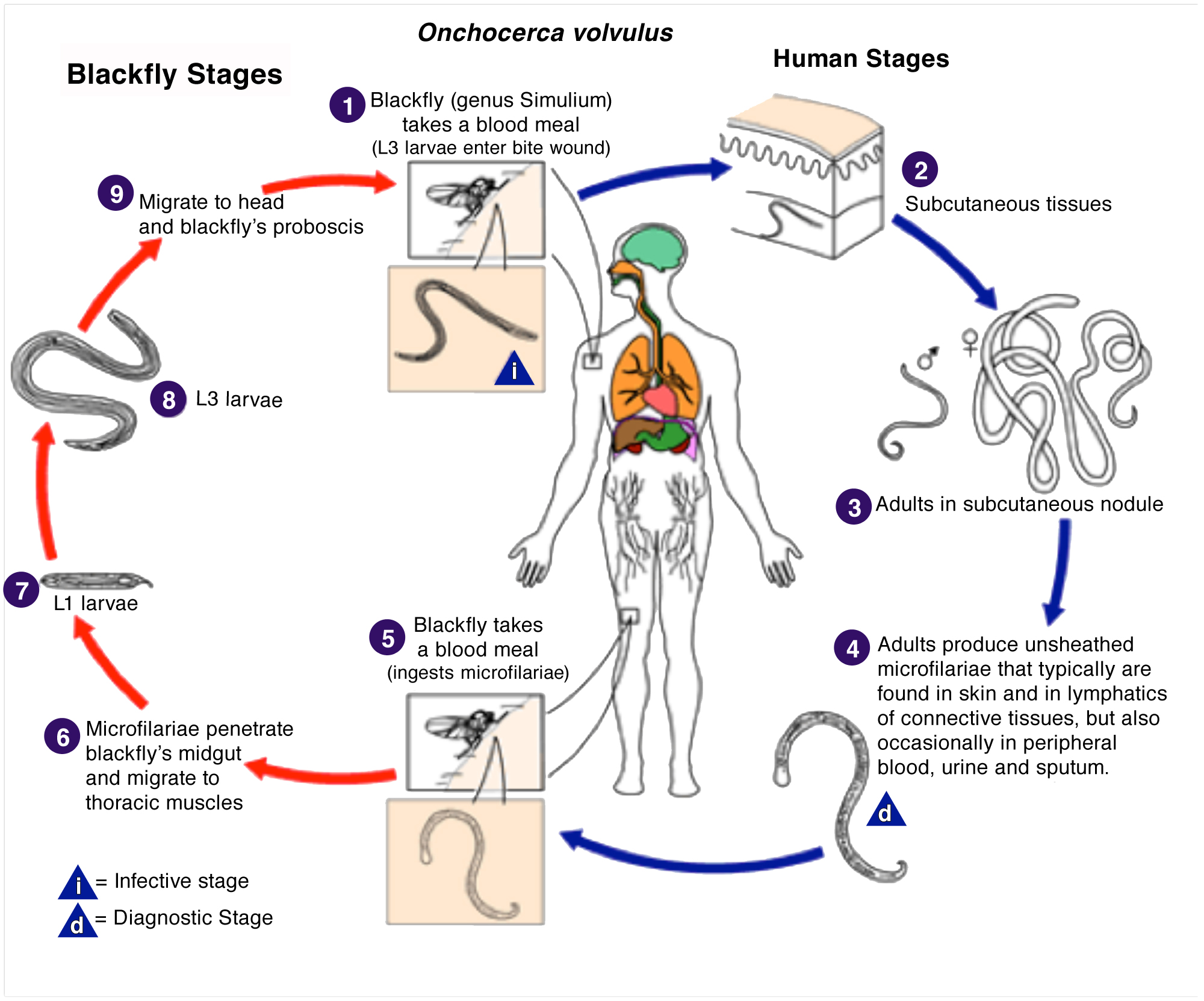
This image is a work of the Centers for Disease Control and Prevention
First Lights
As the extent of onchocerciasis’ renewed grip was becoming evident to WHO scientists in Africa, a scientist named Satoshi Omura had selected fifty Streptomyces bacteria samples with promising insecticidal properties and sent them to the lab at Merck & Co. for further evaluation. Not long afterward, one of the samples began yielding fruit.
An Irish researcher named William C. Campbell, working at Merck’s Institute for Therapeutic Research in New Jersey, discovered remarkable efficacy in one Streptomyces strain versus animal parasites, a strain named Streptomyces avermitilis. This discovery would give rise to an important class of fermentation-based insecticides and endectocides collectively known as avermectins. One compound would ultimately be purified and in its finished form would become known as ivermectin. In 2015, this discovery would earn Omura and Campbell the Nobel Prize in Physiology or Medicine.
This emergence of ivermectin as a powerful tool against onchocerciasis is relatively well known in Public Health lore. But it is only part of the story. As development work on ivermectin was launched, yet another important discovery was being made in Israel by Dr. Yoel Margalith in 1976. After noticing a number of dead mosquito larvae in a standing pool of water in the Negev desert, Margalith gathered a soil sample from which he would eventually isolate a bacteria that would be named Bacillus thuringiensis spp. israelensis, or Bti.
Abbott Laboratories – which had launched a new Bacillus-based biological insecticide for agriculture in 1974, had developed industrial fermentation capabilities and decided to bring Bti – a target-specific biological for mosquitoes and coincidentally black flies – to Public Health programs worldwide. This development had widespread implications for vector management programs that were losing efficacy due to insecticide resistance to traditional chemistries.
As time went on, these two interventions – ivermectin and Bti – would become inexorably linked though the OCP in what is arguably one of the most successful Integrated Vector & Disease Management programs in infectious disease history.
Dynamic Duo
Both ivermectin and Bti took advantage of specific biological pathways/environments that exist within their target pest, but are absent from non-target organisms. This meant that the activity harnessed to control the pest did not also cause collateral damage to unintended organisms/populations. Moreover, ivermectin could be consumed as a therapeutic intervention while Bti would be approved by WHO for application directly into drinking water. While biological controls had already begun establishing a place in agriculture and forestry, the implementation of a biological for BOTH a blood-treatment and an insecticide intervention in Public Health was unprecedented.
Research showed that ivermectin operates by interrupting communication between the helminthic nervous system and musculature of the worm, paralyzing it. The results are specifically harsh on juvenile larvae, 95% of which perish from a single dose. Even though adult worms remain alive, it is the microfilaria that cause the disease. By eliminating the juveniles, the symptoms of onchocerciasis are relieved.
Adult movement is hampered too, which has further implications. When thriving, it is hypothesized that males O. volvulus migrate between nodules where fertile females lie waiting for a mate. But as the males’ movement is hampered by ivermectin, many females go unfertilized.
This spelled good news for the OCP, which had already adopted Bti (VectoBac®) as the foundation of its larviciding program by the mid-80s. Ivermectin therapy showed tremendous promise, but would be greatly undermined unless vectors could be managed as well. To contribute to the program, Abbott developed a new formulation of Bti (VectoBac 12AS) that was designed according to WHO specifications to manage black fly biology and provided major economic concessions on VectoBac through what would eventually turn into a ten-year contract with OCP to provide Bti. The upstart biological insecticide would eventually account for an estimated 80% of OCP’s larviciding program.
The Greatest Gift
Ultimately it would be determined that two doses of ivermectin (Mectizan®) per year were enough to interrupt the onchocerciasis transmission cycle. But how much would it cost for a drug needed to treat millions of people over a minimum 15-year program timeline?
As the story goes, Dr. Peter Vagelos, the acting CEO of Merck & Co., was with his wife at a gathering in 1987 where a short film outlined the scale of the onchocerciasis tragedy. By the next morning, Vagelos, had committed to donating the Mectizan therapy to onchocerciasis victims for free for as long as needed.
This single act would be the paradigm shift for OCP. But now the real fight against onchocerciasis was just getting started. Forgetting, for a moment, the great quantities of Mectizan that would have to be manufactured, the expansive geography under pressure from onchocerciasis covered 400 million people. How does one administer a therapy quickly to such vast numbers over a large geography within developing countries? How would the drug be distributed and managed? All of these questions remained unanswered.
Mectizan® is a registered trademark of Merck & Co.
