Malaria numbers are surging in the Horn of Africa, but not in rural settings.
Djibouti is a small country of around 900,000 inhabitants on the Horn of Africa that rests on the shores of Bab al-Mandab strait, just 20 miles from the coast of Yemen and southwest Asia. Ten years ago, the World Health Organization (WHO) proudly proclaimed that Djibouti was entering the “pre-elimination phase” in its longstanding battle against malaria. Pre-elimination status is reached when a country’s number of confirmed malaria cases approaches zero.
Fast forward to the present and Djibouti is at the opposite end of the spectrum. The tiny nation is at the center of a rapidly growing threat of malaria hypothesized as stemming from the arrival of Anopheles stephensi, an invasive anopheline mosquito species native to Asia and parts of the Arabian Peninsula. After Djibouti experienced only 27 cases of malaria in 2012, an alarming 73,000 cases of malaria were reported in 2020 (see Figure 1). And that may be just the beginning.
Since An. stephensi was first detected in Djibouti in September of 2012, it has gone on to become established in Ethiopia (2018), Sudan (2018), Somalia (2019), and Nigeria (2020). Many fear the invasive vector (which is capable of transmitting both the Plasmodium falciparum and P. vivax malaria parasites) has already spread far wider.
After malaria deaths peaked at 1.8 million in 2004, global efforts were able to reduce malaria mortality by more than two-thirds by the year 2015.
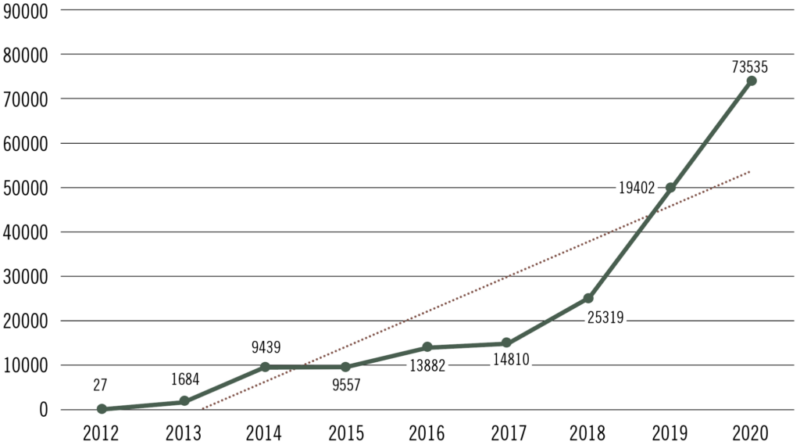
Source: WHO
Unfortunately, that trend has since begun to reverse (see Malaria’s Long Tail, October Public Health Landscape). There are many reasons for this, not the least of which is the growing level of insecticide resistance among disease-vectoring mosquitoes. But regression aside, progress was made chiefly in rural areas where Anopheles gambiae thrives, however, the region is lacking in the technology and experience to control malaria vectors in an urban setting.
Anopheles stephensi is more of an urban dweller. In fact, its bionomics closely resemble that of Aedes aegypti. An. stephensi likes container habitats – plastic tanks, wells, cisterns, barrels, and refuse – and that spells danger for Africa’s urban centers. No less than 70% of Djibouti’s inhabitants live in its capital, and urbanization is a rapidly growing trend across Sub-Saharan Africa. An estimated 40% of the region’s 1.14 billion population now live in urban areas, a number that is expected to rise to 60% by 2050. One study puts the resultant increased risk of malaria from An. stephensi at 126 million inhabitants.1
Stark Numbers
A seminal paper reporting on the arrival of An. stephensi in Djibouti was published by Faulde et al. in 2014. It recounted how surveillance conducted between 2010 and mid-2012 – a yield of 20,434 mosquitoes – turned up no An. stephensi samples. Of the mosquitoes collected, 99.9% were Culex spp., and 0.09% were Aedes aegypti. It wasn’t until September of 2012 that three females of An. stephensi were collected near an animal quarantine station just outside the city. By December, the country’s first malaria outbreak in more than ten years had begun. Between January and May of 2013, more than 1200 malaria cases were reported.2
Faulde notes that officials ramped up vector control efforts. “In response, vector control was initiated in early October using heat fogging of insecticides, larviciding, and habitat sanitation in an effort to mitigate potential malaria transmission risk as well as to prevent further spread of this species. During the 10 months following these vector control measures, no anopheline mosquitoes were detected.”
After the brief respite, however, a second outbreak took hold in December 2013. More than 2000 cases of malaria would be recorded in January and February of 2014. And these outbreaks were different than outbreaks past. The vast majority (more than 80%) of these cases were being reported in urban settings.
Malaria numbers in Djibouti increased steadily as did the number of An. stephensi in traps and larval dippers. By 2016, the number of malaria cases had exceeded 10,000 and by 2017 the presence of An. stephensi was no longer dependent on the rainy season. The invasive species was now being detected year-round.3
In 2019, the number of malaria cases soared to almost 50,000. A field study conducted early that year focused on localized outbreak among a community of some 2,700 French Armed Forces (FAF) living in the city. Epidemiologists were particularly interested in the community because it was known for its fastidious approach to avoiding malaria. The French soldiers wore insecticide impregnated uniforms while on duty and their families slept under Insecticide Treated Nets (ITNs). They routinely applied insect repellent. Nonetheless, 38 cases of malaria were reported among the FAF, demonstrating the adaptability of this new vector.4 Investigations at the military camp found standing water resulting from faulty maintenance of water distribution and drainage.
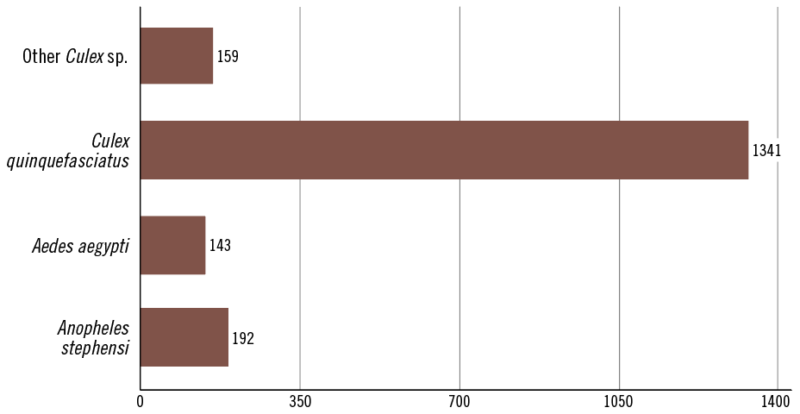
Source: Pommier de Santi et al. cdc.gov/eid (2021)
Data gathered from local traps and water samples around the city provided an eye-opening look at the extent of the problem. A total of 25 different breeding sites were identified, 15 of which contained Anopheles stephensi. Each habitat was artificial and was located in urban or suburban settings (see Figure 2). It was also shown that An. stephensi often shared these habitats with Ae. aegypti, among the most important urban-dwelling vectors in the world.
Anopheles stephensi. Each habitat was artificial and was located in urban or suburban settings (see Figure 2). It was also shown that An. stephensi often shared these habitats with Ae. aegypti, among the most important urban-dwelling vectors in the world.
In 2019, WHO issued a threat notice highlighting the spread of An. stephensi in the Horn of Africa.
A Call to Adapt
These numbers leave little doubt as to the effect of the vector’s spread into Africa. The question now becomes how to respond. The literature surrounding this issue is compelling in its call for rapid reassessment and urgent adaptation of existing vector control programs in the region. An. stephensi has been shown to be resistant to all classes of insecticides and displays behavior that is inconsistent with native African anopheline species. This means that control measures that helped turn the tide with An. gambiae will likely not work with this new, invasive threat.
A 2022 paper published in Malaria Journal asserts that more integrated approaches are necessary, post-haste. “In a recent global analysis, only 8% of the national malaria programs reported having sufficient capacity to implement vector surveillance; and only 28% had capacity to implement larval source management,” writes a team representing the African Leaders Malaria Alliance, Johns Hopkins Center for Communication, and the Ifakara Health Institute. “This study concluded that most countries will not defeat malaria without major investments to improve staffing and boost system-wide capacity…The biting behaviors and low HBIs (Human Blood Indices) in this species suggest that indoor vector control methods such as ITNs may not be the most appropriate. This emphasizes the need for an expanded set of approaches, which may include larval source management programs targeting both Aedes and An. stephensi mosquitoes.”
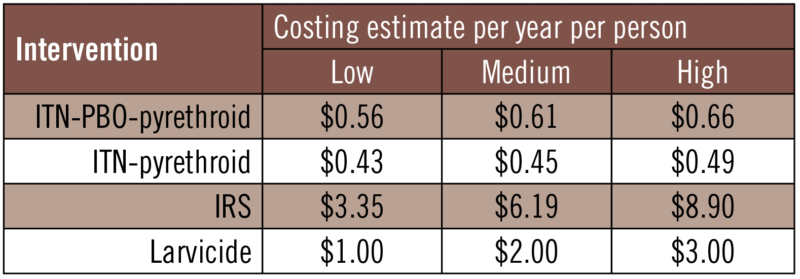
Approximate estimated costs per person per year for the different vector control interventions considered
Source: Pommier de Santi et al. cdc.gov/eid (2021)
Another group citing contributors from the UK, the U.S., and Ethiopia published research in April 2022 in BMC Medicine. In the study, they share results of a predictive model developed to gauge the potential impact of An. stephensi, and transmission of P. falciparum in Ethiopia. The team concludes that the invasion and establishment of An. stephensi in the country may result in a ~50% increase in malaria cases, from 740,000 in 2019 to an estimated 1.13 million. The research also includes an examination of various combinations of interventions to predict impact both from cases averted and from a cost standpoint (see Figure 3).
According to Hamlet et al., “If the mosquito feeds at night and rests inside structures sprayed with IRS, then the widespread use of this intervention alone could be sufficient to mitigate the public health impact. However, if the mosquito was less amenable to indoor vector control, then layering of ITNs and IRS may be insufficient. Within its endemic range, An. stephensi shows a propensity to both crepuscular biting and resting outside of houses compared to African Anopheles species, suggesting a reduced efficacy if it behaves as it does in its endemic range. Compounding this, high density urban locations, where large-scale vector-control campaigns have been historically absent, will present a challenge for establishing ITN access and use as well as achieving sufficient IRS coverage.”
In my experience, there is a tendency in Africa to focus on a lack of funding …
Dr. Silas Majambere
Dr. Silas Majambere is the former Director of Scientific Operations for the Pan-African Mosquito Control Association (PAMCA) and Associate Professor of Medical Entomology, Université des sciences, des techniques et des technologies de Bamako, in Mali. Today, Majambere is part of a team at Valent BioSciences that has collaborated in public and private partnerships with vector control professionals around the world, seeking and sharing knowledge in the control of container mosquitoes. He agrees with these petitions for new thinking, and says he understands that resource challenges can be daunting.
Majambere urges his peers to think outside the box and consider the progress being made in other regions, particularly the great strides that have been made in urban settings for the control of Ae. aegypti for dengue prevention.
“In my experience, there is a tendency in Africa to focus on a lack of funding,” Majambere says. “But this deterrent has become somewhat of a crutch. There will never be enough money for people to do everything they want to do – in vector control or any other discipline. But we have seen that funding can be made available for what is needed.”
Majambere says there are studies, data, and people who can come together and address the Anopheles stephensi challenge, but that those efforts must begin with the realization that vector control in Africa is not a one-sized-fits-all proposition. Progress must start with a commitment to the science and the scaling up of technical training in other parts of the world.
“Unfortunately, the technical expertise required to meet this challenge is still lacking in much of Africa, but that can change,” Majambere says. “We can learn how vector control and disease management has been implemented in the rest of the world – particularly in Europe and the U.S. that have seen great progress. They have seen the impact of LSM and integrated programs in urban and rural settings. This is no longer up for debate.”
Majambere adds that the introduction of Anopheles stephensi into Africa, and the urgency surrounding it, may have a positive effect in the long term.
“In my view, malaria progress in Africa has stalled because it has become dependent on a limited number of interventions that have restricted scope and efficacy,” Majambere says. “They have a role, but this invasive species is not going to be controlled by indoor measures. We have to accept that. We have to adapt, and when we do, we will do better in rural settings as well.”
1. “A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk.” Sinka et al. PNAS (2020), Vol. 117, No. 40. https://doi.org/10.1073/pnas.2003976117
2. “First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa.” Faulde et al. Acta Tropica 139 (2014) 39-43 http://dx.doi.org/10.1016/j.actatropica.2014.06.016
3. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established—malaria emerging” Seyfarth et al. Parasitology Research (2019) 118:725–732; https://doi.org/10.1007/s00436-019-06213-0
4. “Role of Anopheles stephensi Mosquitoes in Malaria Outbreak, Djibouti, 2019” Pommier de Santi et al. Emerging Infectious Diseases (2021) Vol 27, No. 6
5. “Anopheles stephensi in Africa requires a more integrated response.” Mnzava et al. Malaria Journal (2022) 21:156 https://doi.org/10.1186/s12936-022-04197-4
6. “The potential impact of Anopheles stephensi establishment on the transmission of Plasmodium falciparum in Ethiopia and prospective control measures.” Hamlet et al. BMC Medicine (2022) 20:135; https://doi.org/10.1186/s12916-022-02324-1
Image: Jim Gathany, via Wikimedia Commons
