vector control
Mamy Ingabire: Transforming Vector Control in Africa
Mamy Ingabire is an entrepreneur dedicated to using cutting-edge technology to address critical challenges across various industries.
As the Managing Director of Charis UAS, Rwanda’s first licensed drone company, she has played a fundamental role in advancing the use of Unmanned Aerial Vehicles (UAVs) to improve efficiency in vector control, agriculture, construction, mapping, healthcare, and more. Under her leadership, Charis UAS has leveraged drone technology to revolutionize data collection and digital solutions.
The Drone Edge in Vector Control
Achieving global vector control’s potential requires “realigning programs to optimize the delivery of interventions that are tailored to the local context [and]…strengthened monitoring systems and novel interventions with proven effectiveness.” This includes “integration of non-chemical and chemical vector control methods [and] evidence-based decision making guided by operational research and entomological and epidemiological surveillance and evaluation.”
Drones or “unmanned aerial vehicles” (UAVs) can save time and money compared to conventional ground-based surveys. Sophisticated models and monitoring equipment can be purchased for a few thousand dollars. They don’t require a pilot’s license, they are becoming easier to fly, and their paths can be fully automated through AI, machine learning, global positioning systems, and computer vision.
Mosquito Control Drone Application
Check out this video from Calcasieu Parish Mosquito and Rodent Control on how they are using drone technology to spray in hard to reach areas and increase the efficiency of eliminating disease-carrying populations of mosquitos.
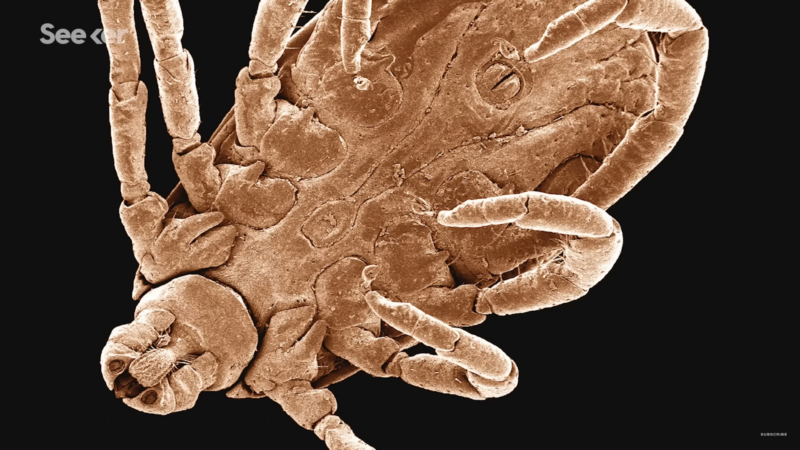
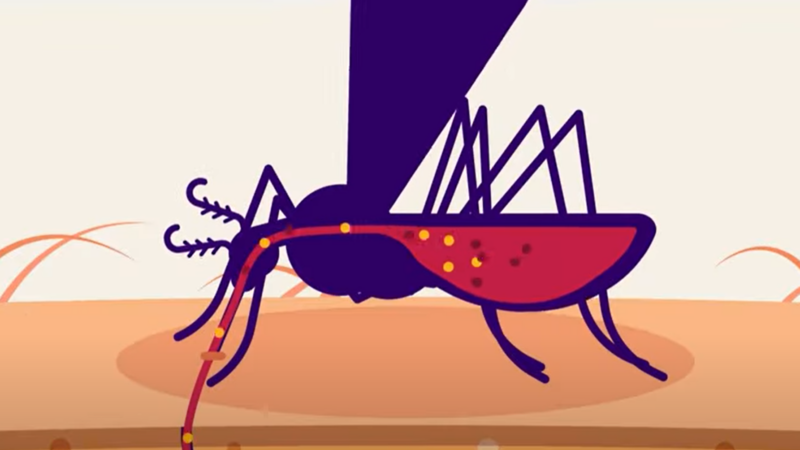
What Does Lyme Disease Do to Your Body?
What exactly is the connection between a tick bite and lyme disease?
While we’re not sure exactly where and when the disease originated, we do know a lot about how it works, its signs, its symptoms in humans and dogs, how it’s spread and its treatment.
Check out this video by Seeker to learn all about what lyme disease does to your body.
Dengue Explained in Five Minutes
Check out this video by Free Med Education to learn about dengue fever, its cause, and its symptoms.
The Rise of Dengue: A Global Perspective
Around the world, dengue is considered the most common viral disease transmitted by mosquitoes that affects people. According to the World Health Organization (WHO), the disease is now endemic in more than 100 countries. In the first three months of 2024, over five million dengue cases and over 2000 dengue-related deaths were reported globally. The figures so far project that 2024 could be even worse than 2023, with the regions most seriously affected being the Americas, South-East Asia, and Western Pacific.
In the Americas, there were 6,186,805 suspected cases of dengue reported in the first 15 weeks of 2024. To put this into perspective, according to the Pan American Health Organization (PAHO), this figure represents an increase of 254% compared to the same period in 2023. Of these cases 5,928 were confirmed and classified as severe dengue.
Peter DeChant: Vector Control Visionary
The story of Peter DeChant, a veteran in the mosquito and vector control profession whose journey spans over four decades, is one of dedication, innovation, and relentless commitment to combating mosquito-borne diseases.
Peter’s journey began in 1978 when he became a field technician with Multnomah County Vector Control in Portland, Oregon. Little did he know then that this would mark the start of a lifelong crusade against one of the deadliest creatures on the planet.
By 1983, Peter’s skills and passion for his work led him to the role of Chief Sanitarian, where he led the program for 14 years. It was during this time that he honed his expertise and laid the foundation for his future endeavors.
The Economics of Resistance
It would be extremely difficult to calculate, with any high degree of accuracy, the global economic impact of insecticide resistance. For starters, we must consider that insect management plays a pivotal role in a variety of sectors – agriculture, home and garden, forestry, structural applications, and vector control. Analysis of the totality of economic impacts arising from resistance in any one of these sectors quickly becomes a complicated interplay of variables that interact within that given system.
To account for the full economic impact, one must layer in the amount being spent on insect management and how much of that investment is lost to resistance, but also the economic impact of losses to the overarching objectives of a given program.
To calculate the impact, you must first calculate what is at risk.
Birds on the Brink
Hardly anyone visits the desolate outpost of Coldfoot, one of Alaska’s few communities outside the Arctic Circle accessible by road. Its 34 residents live in rustic accommodations along the Dalton Highway. The town’s highlights include an inn, a café, a gas station and a basic airport with a gravel landing strip. All day long, 18-wheeler fuel trucks thunder by on supply runs between Fairbanks and the oil fields of Prudhoe Bay further north. Some will stop to eat and tank up at Coldfoot because the next human habitation is 234 miles away, a town grimly named Deadhorse.
They say Coldfoot got its name from the days of the 1900 Gold Rush when miners would come as far as this remote settlement before getting “cold feet” and turning back. It’s still a lonely place, but one unexpected visitor showed up recently inside an infected Swainson’s thrush (Catharus ustulatus): the avian malaria parasite, Plasmodium circumflexum.
In 2011, scientists tested 676 birds representing 32 resident and migratory bird species captured from three northern locations in Alaska: Anchorage (61°N), Fairbanks (64°N) and Coldfoot (67°N). In Anchorage and Fairbanks, they found 49 birds infected by Plasmodium parasites. In Anchorage, even resident birds and hatchlings of species such as the boreal chickadee (Poecile hudsonicus), the varied thrush (Zoothera naevia) and the fox sparrow (Passerella iliaca) were found infected. The parasite was also detected in black-capped chickadees (Poecile atricapillus) and a myrtle warbler (Dendroica coronata coronata) in Fairbanks, indicating that transmission had occurred locally.
The Economics of Malaria
In the 2022 World Malaria Report, compiled by the World Health Organization (WHO), the total spend on funding the fight of malaria in 2021 was estimated at USD 3.5 billion. Over that year, the same report states that there were an estimated 247 million cases of malaria and 619,000 malaria deaths globally.
More recently, over the course of the 20th Century, malaria is believed to have claimed between 150–300 million lives. The disease is contracted predominantly in the tropical regions: sub-Saharan Africa, Asia and the Amazon basin. This is due to the prevalence of the Anopheles mosquito that transmits the disease. Poorer regions of Africa bear the vast majority of the burden. In 2021, around 95% of the diagnosed cases and deaths were on the African continent, 80% of which were children under the age of five. The disease is entirely preventable and curable with prompt diagnosis and effective methods of treatment which require sufficient investment and funding.