The burden of vector-borne diseases (VBDs) is one of public health’s most pressing challenges. VBDs are caused by pathogens such as arboviruses (arthropod-borne virus), bacteria, and parasites that are transmitted to humans and animals through the bites of infected arthropods including mosquitoes, ticks, sandflies, and fleas, among others. According to the World Health Organization (WHO) , “vector-borne diseases account for more than 17% of all infectious diseases, causing more than 700,000 deaths annually worldwide”.
Beyond these broad statistics, attempts to quantify the global burden of VBDs is extremely challenging – for a number of reasons. At the highest level, even “burden” has an underlying complexity in public health terms: burden may refer to the number of cases of a given disease as well as the number of deaths.
Burden can also represent Disability-adjusted Life Years (DALYs), a measure that accounts for the long-term effects of disability among the afflicted, as well as the economic impact of disease from regions and countries all the way down to households and individuals. These economic impacts can be further scrutinized as reduced productivity among the populace, increased healthcare costs, and negative impacts on tourism; all of which can directly affect the GDP and economic growth of local and regional economies. And that’s just the beginning.
External factors affect data studies as well. Accurate and comprehensive data on the prevalence, incidence, and mortality rates of vector-borne diseases are often lacking, particularly in low-income countries with limited resources for surveillance and reporting. This makes it difficult to assess the true extent of the problem. Moreover, many vector-borne diseases are vastly underreported due to asymptomatic cases, misdiagnosis, or lack of access to healthcare. This can also lead to an underestimation of the actual impact of these diseases.
Now add climate and environmental factors to the mix. Vector-borne diseases are influenced by a wide range of factors, including climate, land use, and urbanization. These factors can vary greatly over time and across different regions, making it difficult to predict and quantify the overall impact. One must then account for disease dynamics and interactions, as vector-borne diseases often have complex transmission cycles involving multiple hosts, vectors, and pathogens. Understanding these interactions is crucial for assessing the overall impact but can be challenging due to the dynamic nature of these relationships.
The effectiveness of control and prevention measures adds yet another layer of complexity. The impact of vector-borne diseases can be mitigated by various control and prevention measures such as larval source management, the use of biological and chemical insecticides, repellents, bed nets, and vaccines. But the effectiveness of these interventions can vary by region and over time, further complicating the estimation of global impact. This is particularly true with the onset of resistance to many traditional chemical controls.
… vector-borne diseases account for more than 17% of all infectious diseases, causing more than 700,000 deaths annually worldwide.
World health organization
And, while we are making progress against some VBDs (e.g., malaria), others, such as dengue, are becoming more of a problem. New vector-borne diseases can emerge, and previously controlled diseases can re-emerge due to changes in environmental conditions, human behavior or other factors.
All of these factors and the dynamic landscape makes quantification of the total global impact of VBDs virtually impossible.
The One Constant
There is one thing we can say with certainty, however. The burden of VBDs is disproportionately borne by poor and marginalized communities – populations living in low- and middle-income countries where access to adequate healthcare and prevention measures is limited. Places where poor-quality housing is the norm, and basic sanitation and running water the exception. Locations where resources for disease surveillance and diagnostic testing may be lacking or nonexistent. Whatever the total global burden of vector-borne disease may be, there is no doubt it is felt most acutely in these impoverished areas.
So, in the absence of any mechanism to quantify the total burden, we are left to examine the parts that make up the global whole. Let us explore the most important vector-borne diseases through the lenses of mortality, morbidity, and economic burden. Here is a short list.
Chikungunya (CHIKV)
Vector(s): Aedes aegypti and Aedes albopictus mosquitoes; Pathogen: Arbovirus
Geographies at Risk: Countries most impacted by Chikungunya include India, countries in the Indian Ocean region (such as Réunion Island, Mauritius, and the Maldives), countries in Southeast Asia (such as Indonesia, the Philippines, and Thailand), and nations in the Americas (such as Brazil, Colombia, and the Caribbean countries).
Impact Summary: Chikungunya is not considered a fatal disease – the overall mortality rate is low, typically ranging from 0.1% to 1% in various outbreaks. Most cases resolve within a few weeks. However, 25–40% of those afflicted with Chikungunya develop chronic musculoskeletal and arthritic symptoms which may lead to permanent joint damage and chronic joint pain. These long-term effects are most closely associated with the disease. In fact, the word Chikungunya comes from the African Makonde language and means “bent over in pain.”
A 2021 study led by researchers from the Stanford University School of Medicine estimated that from 2010 to 2019, CHIKV caused an average annual global loss of over 106,000 DALYs.1 Economic losses and costs may arise from direct medical expenses, lost productivity due to short-term and chronic illness, and negative effects on tourism. During the 2005-2006 CHIKV outbreak on Réunion Island, for example, the epidemic was estimated to have cost about $52.3 million – 60% in direct costs and 40% in indirect costs.2

Dengue
Vector(s) Primarily Aedes aegypti, also Aedes albopictus; Pathogen: Arbovirus
Geographies at Risk: Regions most impacted by dengue include Southeast Asia, the Western Pacific, the Americas, and Africa. Some of the most affected countries include Brazil, India, the Philippines, Indonesia, Vietnam, Bangladesh, Mexico, and Thailand.
Impact Summary: Dengue fever has become a major public health concern in many regions and continues to increase in impact. According to the World Health Organization, about half of the world’s population is now at risk of dengue. One modeling estimate indicates there are around 390 million dengue infections per year, worldwide – a 30-fold increase in dengue incidence over the past 50 years.3 Insecticide resistance has been cited as a leading cause for recent dengue increases in Asia (see Resistance in the Limelight, PHL, February, 2023).
Dengue can be fatal if not treated properly, especially in severe cases. The global mortality rate for dengue is estimated to be around 1% for hospitalized cases, but it can be reduced to below 0.1% with proper case management. The World Health Organization (WHO) estimates that dengue causes approximately 10,000 to 20,000 deaths per year. According to the Global Burden of Disease Study , dengue was responsible for approximately 2.38 million DALYs in 2019.4 Economically speaking, dengue has a sweeping impact on healthcare systems, productivity, and tourism. A study published in 2016 estimated the total annual global cost of dengue to be around $8.9 billion, including both direct medical costs and indirect costs.5

Chagas Disease
Vectors(s): Triatomine bugs “kissing bugs;” Pathogen: Trypanosoma cruzi (protozoan parasite)
Geographies at Risk: Chagas disease primarily affects countries in Latin America, where triatomine insects are endemic. Some of the most impacted countries include Brazil, Argentina, Bolivia, Mexico, Colombia, Venezuela, Paraguay, Ecuador, Honduras, and El Salvador.
Impact Summary: Chagas disease is transmitted primarily through the feces of infected triatomine insects, which ingest the parasite through the blood of an infected animal. After feeding, kissing bugs defecate on the skin and deposit the parasites which then enter the body of a new host through the eyes, mouth, or a wound from the bug’s bite. Dozens of biting Triatominae species act as vectors and hundreds of mammalian species including humans, rodents, canines, and nonhuman primates, both wild and domesticated, act as reservoirs for the disease. While the disease is mostly concentrated in Latin America, increased global travel and migration have led to cases being reported in the United States, Canada, Europe, and other regions.
Chagas disease can be fatal if left untreated, particularly in its chronic phase, which can lead to heart failure or sudden death. The World Health Organization (WHO) estimates that Chagas disease causes approximately 12,000 deaths per year globally. However, this figure is likely underestimated due to underdiagnosis and underreporting. According to the Global Burden of Disease Study, there were approximately 6.5 million cases of Chagas Disease in 2019, responsible for about 275,00 DALYs.6 While no studies on the economics of Chagas Disease have been conducted in the past ten years, a 2013 study estimated a global economic burden of between $7 and $19 billion.7
CDC
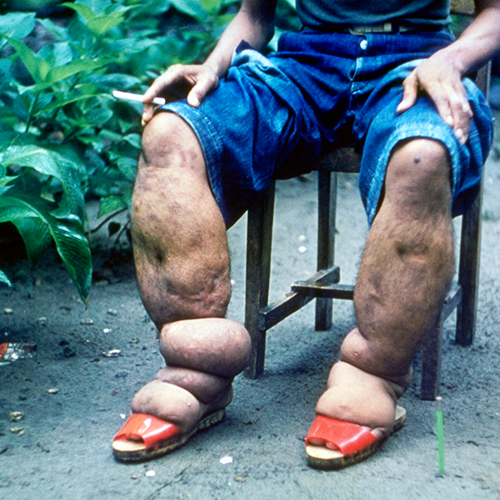
Lymphatic filariasis
Vector(s): Wide range of mosquito species, primarily Culex quinquefasciatus in the Americas, Anopheles, in Africa, and Aedes in Asia; Pathogen(s): Three species of parasitic roundworms including Wuchereria bancrofti (90% of all cases), Brugia malayi, and Brugia timori.
Geographies at Risk: Lymphatic filariasis primarily affects countries in tropical and subtropical regions of Africa, Asia, the Pacific Islands, and the Americas. Some of the most impacted countries include India, Nigeria, Indonesia, Bangladesh, Democratic Republic of Congo, Mozambique, Philippines, Myanmar, Côte d’Ivoire, and Brazil.
Impact Summary: Lymphatic filariasis can cause lymphedema (tissue swelling, usually in the arms or legs) and elephantiasis. While it is not considered a fatal disease (there is no precise global estimate for the number of deaths attributed directly to the disease), it can cause significant disability, disfigurement, and social stigma. According to World Health Organization, the Global Burden of Disease Study, lymphatic filariasis was responsible for approximately 1.63 million DALYs in 2019.8
The economic impact of lymphatic filariasis is substantial in affected countries. In addition to costs stemming from direct medical expenses and disability, the loss of productivity due to social stigma is pronounced with this disease. The total economic impact of lymphatic filariasis is difficult to calculate, but a 2021 study estimated the annual global economic loss in India alone approaches $1 billion.9
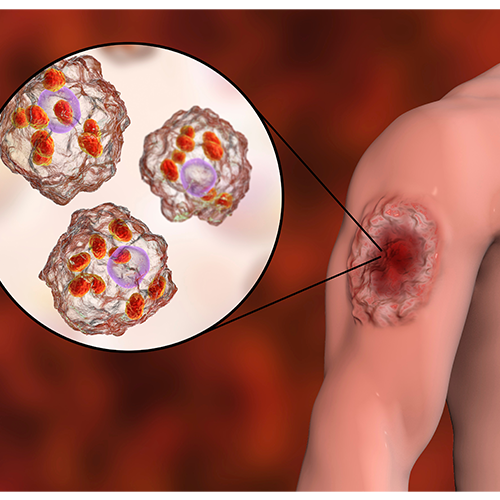
Leishmaniasis
Vector(s): More than 30 species of sandflies; Pathogen(s): More than 20 species of the genus Leishmania (protozoan parasites)
Geographies at Risk: Leishmaniasis primarily affects countries in tropical and subtropical regions, including parts of Africa, Asia, the Americas, and southern Europe. Some of the most impacted countries include India, Bangladesh, Nepal, Brazil, Ethiopia, Sudan, Somalia, Kenya, and Afghanistan.
Impact summary: Leishmaniasis is a vector-borne disease transmitted by female sandflies and caused by microparasites that grow and reproduce within living cells of their hosts. There are three main forms of the disease: cutaneous (affecting skin), mucocutaneous (affecting mucous membranes), and visceral (affecting internal organs). The latter primarily affects children under five years of age and is commonly associated with malnutrition or other forms of immunosuppression such as HIV.10
The World Health Organization estimates that there are 12 million people infected with leishmaniasis, with between 0.9 and 1.6 million new cases each year. Of the 20,000 and 30,000 deaths attributed to the disease, most of which are attributed to visceral leishmaniasis. If left untreated, the mortality rate of this form of the disease is greater than 90%. According to the Global Burden of Disease Study, leishmaniasis was responsible for approximately 675,000 DALYs in 2019.11
Leishmaniasis is a disease that affects the most impoverished areas of developing countries, and as such is considered grossly underreported. As a result, the literature does not include any studies on the global economic impact of the disease. However, one study conducted in 2016 by researchers from the Department of Medical Microbiology at the University of Manitoba, Canada, found that as much as 72% of a household’s income can be lost to a single case of leismaniasis.12
Dr. Catherine L.E. Craker
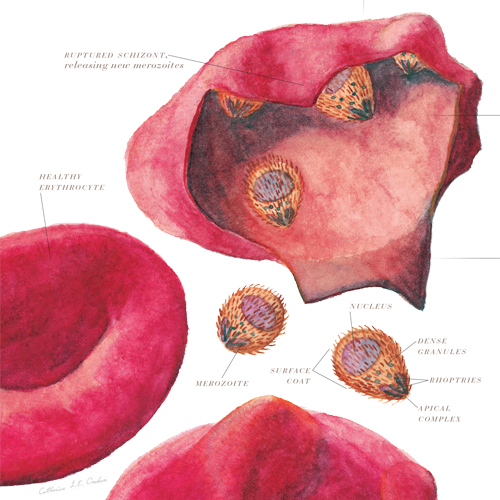
Malaria
Vector(s): Anopheles mosquitoes; Pathogen(s): Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae (protozoan parasites)
Geographies at Risk: Tropical and subtropical regions, including parts of Africa, Asia, Central and South America, and the Pacific Islands. Some of the most impacted countries include Nigeria, Democratic Republic of Congo, Mozambique, Uganda, Niger, Burkina Faso, Ghana, Mali, Angola, and India.
Impact Summary: Malaria is the best known of all vector-borne diseases, for all the wrong reasons. Malaria accounts for the vast majority of deaths among all vector-borne diseases, with children under the age of five accounting for about 80% of those deaths. As with many vector-borne diseases, poverty plays a significant role in transmission. In 2020, the WHO African Region was home to 95% and 96% of malaria cases and deaths, respectively.13 While significant progress was made in reducing malaria deaths since 2000, that progress has stalled in recent years. After achieving a 35% reduction in malaria deaths by 2015, deaths in Africa have since increased every year (see Malaria’s Long Tail, PHL, October, 2022).
According to the 2021 World Malaria Report, there were an estimated 241 million malaria cases and 627,000 malaria deaths worldwide in 2020. The 2019 Global Burden of Disease Study puts the global malaria burden at a staggering 46.2 million DALYs.14 From an economic standpoint, malaria is estimated to cost at least $12 billion annually in Africa alone, and as much as $42 billion was spent in global anti-malaria efforts between 2000 and 2016.

Narda Lebo
Onchocerciasis
Vector(s): Six species of blackflies from the genus Simulium; Pathogen: Onchocerca volvulus (parasitic roundworm)
Geographies at Risk: Primarily affects sub-Saharan Africa, with lesser incidence in some parts of Latin America and Yemen. Some of the most impacted countries include Nigeria, Democratic Republic of Congo, Ethiopia, South Sudan, Cameroon, Uganda, Ghana, Chad, Guinea, and Burkina Faso.
Impact Summary: The mortality rate for onchocerciasis is low and there is no precise global estimate for the number of deaths attributed directly to the disease. Its primary effect is the loss of sight amongst infected adults given colonization of parasitic worms in the eyes. As another vector-borne disease closely linked to impoverished areas, 99% of those afflicted with onchocerciasis are in Africa. Curbing the impact of onchocerciasis, or “river blindness,” is one among the most successful of all global public health efforts, however, as public and private partnerships were able to reduce the number of DALYs attributable to the disease by approximately 86% between 1995 and 2015 (see It All Started with a Gift, PHL, August, 2021).
Since the ocular damage caused by advanced stages of the disease is often irreversible, the current number of global DALYs lost to onchocerciasis is still in the 1.15 million DALY range. While precise global estimates of the economic burden of onchocerciasis are limited, a systematic review of onchocerciasis studies conducted in 2019 found that eliminating the disease would generate billions in economic benefits.15

Scott Bauer, USDA ARS
Lyme Disease
Vector(s): Ixodes scapularis (black-legged tick) and Ixodes pacificus (Western black-legged tick); Pathogen(s): Borrelia burgdorferi and rarely, Borrelia mayonii (bacteria)
Geographies at Risk: Primarily temperate regions of North America, Europe, and Asia. Some of the most impacted countries include the United States, Canada, Germany, France, United Kingdom, Poland, Sweden, Finland, Austria, and Switzerland.
Impact Summary: Quantifying the global impact of Lyme disease is particularly challenging due to underreporting and misdiagnosis. For example, a recent study conducted by researchers at Columbia University estimated the number of actual cases of Lyme Disease in the Northeastern U.S. is likely 10 times greater than what is reported.16
Deaths directly attributed to Lyme disease are rare. However, it can cause significant disability and long-term complications if not treated promptly. One of the long-term effects of Lyme disease is a weakened immune system, which can lead to a heightened susceptibility to other diseases. Given the reporting challenges along with variations in incidence differences in healthcare systems, there are no global estimates of DALYs for Lyme disease. However, a 2015 study in the Netherlands estimated the burden of Lyme disease in that country to be around 1,749 DALYs per year among a population of 16.6 million.17 A 2022 study in the United States estimated the annual cost of Lyme disease to approach $1 billion.18
Zika
Vector(s): Infected Aedes mosquitoes, particularly Aedes aegypti; Pathogen: Arbovirus
Geographies at Risk: Zika virus disease has been reported in countries across Africa, the Americas, Asia, and the Pacific Islands. However, the most significant outbreaks and impact occurred in the Americas, especially in 2015 and 2016. Some of the most impacted countries include Brazil, Colombia, Venezuela, Mexico, Honduras, El Salvador, Puerto Rico, Dominican Republic, and French Martinique.
Impact Summary: Like many other vector-borne diseases, often mild symptoms resulting in underreporting makes quantifying the global impact of Zika virus disease challenging. While it is not considered a fatal disease, it can cause severe complications, such as microcephaly in infants born to infected mothers, and neurological complications such as Guillain-Barré syndrome in adults.
The most significant outbreak of Zika occurred in 2015-2016, and impact estimates apart from that outbreak are scarce. While year-on-year estimates of DALYs lost to Zika are relatively low, a recent study estimated the burden of Zika virus disease in Latin America and the Caribbean during the 2015-2016 outbreak to be around 103,000 DALYs, with nearly half of that number centered in Brazil.19 An economic impact study conducted by researchers at Johns Hopkins in collaboration with the United Nations Development Program and the Red Cross estimated the total cost of the outbreak in South American and the Caribbean to fall somewhere between $7 and $18 billion.

West Nile Virus
Vector(s): Culex pipiens, Culex tarsalis, and Culex quinquefasciatus mosquitoes; Pathogen: Arbovirus
Geographies at Risk: West Nile virus (WNV) is found in many regions across the globe. Historically, countries in Africa, Europe, the Middle East, North America, and West Asia have reported WNV cases and outbreaks.
Impact Summary: Once again, quantifying the global impact of West Nile virus is challenging due to underreporting and the often mild or asymptomatic nature of the disease. In fact, the Centers for Disease Control and Prevention estimates that between 70%-80% of WNV cases are asymptomatic.20 But while most WNV infections are mild, severe cases can lead to encephalitis, meningitis, or death. The WHO reports that approximately 1 in 150 WNV infections results in severe neurological disease, with a case fatality rate between 4% and 14% among hospitalized patients.
A review of the literature did not yield any data on global estimates of DALYs lost to West Nile virus. However, it is known that the disease can cause long-term neurological complications and disability in those who experience severe illness, which would contribute to its DALY burden. While global economic impact data for WNV is also lacking, a 2014 study appearing in the American Journal of Tropical Medicine and Hygiene estimated the total cost of WNV disease in the US from 1999 to 2012 to be approximately $778 million.21
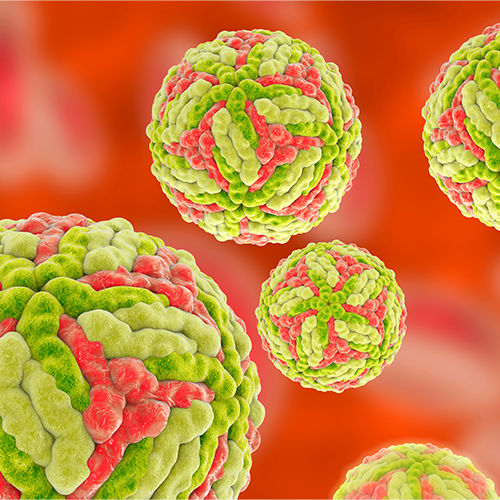
Japanese Encephalitis
Vector(s): Culex species mosquitoes, particularly Culex tritaeniorhynchus; Pathogen: Arbovirus
Geographies at Risk: Japanese encephalitis (JE) is prevalent in East and Southeast Asia, as well as some parts of the Western Pacific. The countries most affected by JE include China, India, Nepal, Vietnam, Cambodia, Bangladesh, Indonesia, Laos, Myanmar, and the Philippines.
Impact Summary: Japanese encephalitis is an important and potentially fatal disease that can cause severe infections. It is the single largest cause of viral encephalitis in Asia. According to the World Health Organization, approximately three billion people live in JE risk areas of JE and there are an estimated 68,000 clinical cases of JE annually. Between 13,600 and 20,400 deaths, occur each year. JE fatality rates range from 10–20%, while 20–30% of the survivors have permanent neurological disorders, between 30% and 50% of which experiencing significant cognitive, behavioral, or motor disabilities.22
Given its long-term effects, the relative DALY to incidence ratio for Japanese encephalitis is high. A 2011 study estimated the global burden of JE to be between 265,000 and 1.86 million DALYs as of 2005. Global estimates of the economic burden of JE are limited. However, a 2021 study examining the economic burden of JE in just the Chinese province of Zhejiang exceeded $12 million in a 5-year period beginning in 2013.23

Muhammad Mahdi Karim
Yellow Fever
Vector(s): Aedes or Haemagogus species mosquitoes; Pathogen: Arbovirus
Geographies at Risk: Yellow Fever is endemic in tropical areas of Africa and South America. The countries most affected by yellow fever include Nigeria, Democratic Republic of the Congo, Angola, Uganda, Brazil, Colombia, Peru, Bolivia, Venezuela, and Ghana.
Impact Summary: Yellow Fever is related to West Nile, St. Louis encephalitis, and Japanese encephalitis viruses. It can be transmitted by mosquitoes from monkeys to humans when humans are visiting or working in the jungle, or to humans from an infected human who brings the virus into an urban setting.24
Yellow Fever is a particularly dangerous vector-borne disease. WHO estimates there are between 84,000 to 170,000 cases of yellow fever annually, resulting in 29,000 to 60,000 deaths. The case fatality rate for severe yellow fever cases is between 20-50%. According to the Global Burden of Disease Report, the global burden of Yellow Fever 290,000 DALYs in 2019. The disease’s impact on DALYs is significant, considering its high mortality rate and potential for large-scale outbreaks, most of which take place in West Africa. A 2017 study estimated that during the 2016 yellow fever outbreak in Angola, the total economic burden was around $127.7 million, including direct medical costs, lost productivity, and vaccination campaign expenses.25 Underreporting remains a significant challenge in compiling accurate Yellow Fever data.
Strategies to Reduce the Global Burden of VBDs
Just as unpacking burden dynamics is a complex process, reducing the risk of vector-borne diseases requires a multifaceted approach that involves various stakeholders, including government agencies, international organizations, local communities, and individuals. The best strategies and critical success factors for reducing the risk of vector-borne diseases include:
Surveillance and monitoring: Establishing and maintaining robust disease and vector surveillance systems to detect and monitor outbreaks, track disease trends, and guide intervention strategies.
Vector control: Implementing effective vector control measures, such as environmental management (e.g., eliminating breeding sites) larviciding, insecticide-treated bed nets (for malaria), indoor residual spraying, and biological control methods.
Vaccination: Developing and deploying vaccines for preventable vector-borne diseases, such as yellow fever and Japanese encephalitis, and ensuring high vaccination coverage in at-risk populations.
Public health education: Raising awareness about vector-borne diseases, their transmission, and prevention measures among the public, health workers, and policymakers. This includes promoting personal protective measures, such as using insect repellent, wearing protective clothing, and installing screens on doors and windows.
Access to healthcare and early diagnosis: Ensuring timely access to healthcare services for early diagnosis and treatment of vector-borne diseases. This can help reduce morbidity and mortality, as well as prevent further transmission.
Integrated vector management (IVM): Adopting a comprehensive approach to vector control that considers the local context, available resources, and ecological factors. IVM strategies may include combining chemical, biological, and environmental control methods to maximize their impact.
Intersectoral collaboration: Collaborating across various sectors, including health, environment, agriculture, and urban planning, to address the social and environmental determinants of vector-borne diseases.
Research and development: Investing in research to better understand vector-borne diseases, their transmission dynamics, and the effectiveness of interventions. This includes developing new tools for vector control, diagnostics, and treatments, as well as improving existing ones.
Capacity building: Strengthening the capacity of healthcare systems and public health agencies to effectively respond to vector-borne disease outbreaks and implement prevention measures.
International cooperation: Collaborating with international partners, such as the World Health Organization, to share best practices, exchange information, and coordinate efforts to combat vector-borne diseases.26
*References can be found here.